
What is pH and how is it measured?
pH, in its simplest form is a measurement how acidic or alkaline a solution is. In other words, it describes the concentration of hydrogen ions (H+) or hydroxyl ions (OH-) within the solution; the pH scale allows you to define this.The pH scale runs from 0 to 14. From a pH of 0 to less than 7, solutions are considered acidic meaning H+ ions are present in a greater concentration. At a pH of 7, a solution is neutral and has equal H+ and OH- ions. From a pH of greater than 7 up to the maximum of 14, the solution is alkaline and has a greater concentration of OH- ions. The scale is logarithmic, meaning a difference of 1 pH actually means a tenfold difference in acidity/alkalinity.
In terms of water testing, monitoring pH is essential as it can have large impacts on aquatic organisms. However it is not only the water industry that would measure pH. In food manufacturing for example, pH is often measured as it can have an impact on taste and quality of the finished goods.
Check out our pH introduction video below:
What are the different ways of testing for pH?
There are many options available when it comes to pH testing, differing from each other in ways such as difficulty, speed, accuracy and price. With this variety, you will be sure to find the option to suit your requirements.





pH test strips and papers are a quick and simple option. They come in the form of single use strips or reels that change colour to indicate the pH. The simplest type of pH test paper would be litmus paper, simply indicating if your test sample is acidic or alkaline. If you would like to read more on the differences between pH paper reels and test strips, you can check out our blog post here.
pH indicator solution will exhibit a colour change depending on the pH of the sample they are being used to test. Indicator solution can be useful in testing solids for exmple soil.
Reagents are added to your sample where a colour change is exhibited. This is then compared to a chart or a colour wheel standard by eye to give the pH result. These are often limited to only being suited for clear liquids. An example would be a comparator.
Photometers and colorimeters build on the colour test kits. Reagents are added to your sample exhibiting a colour change however this is evaluated by an electronic machine giving you read out of the result. These are often limited to only being suited for clear liquids.
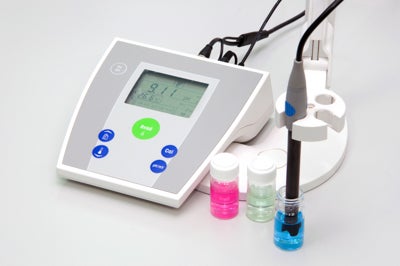
Electrochemistry pH meters work using a pH electrode which measures the H+ concentration in your sample. The electrode is connected to a meter which gives you the result usually as a digital display and can have accuracy as high as 0.001 pH.
Request information from a technical specialist
Camlab have a number of trained technical specialists on hand to help with any enquiry you may have. You can request more information or a call back using the button below. Please try and provide as much information as possible.

